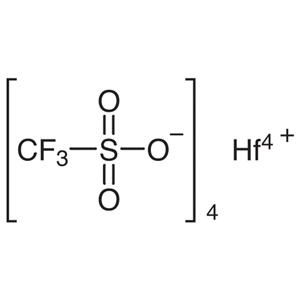
Hafnium(IV) Trifluoromethanesulfonate
- Product code: ICTT1708
- Purity/Analytical Methods: >90.0%(T)
Basic Information
| Appearance and Shape (20°C) | Solid |
|---|---|
| CAS RN | 161337-67-3 |
| MDL number | MFCD01321255 |
| Molecular weight | 774.74 |
| Molecular Formula | C4F12HfO12S4 |
| Product code | ICTT1708 |
| PubChem Substance ID | 125310596 |
| Purity/Analytical Methods | >90.0%(T) |
| Reaxys-RN | 11552495 |
| Situations to avoid | Hygroscopic |
| Store under inert gas | Store under inert gas |
Technical Specifications
Technical Specifications
| Appearance | White to Light yellow powder to crystal |
|---|
Safety and Regulatory
GHS
| Pictograms |
|
|---|---|
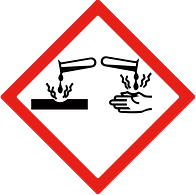
| Hazard statement | H314 : Causes severe skin burns and eye damage. |
| Precautionary Statements | P501 : Dispose of contents/ container to an approved waste disposal plant P260 : Do not breathe dusts or mists P264 : Wash skin thoroughly after handling P280 : Wear protective gloves/ protective clothing/ eye protection/ face protection P303 + P361 + P353 : IF ON SKIN (or hair): Take off immediately all contaminated clothing. Rinse skin with water/shower P301 + P330 + P331 : IF SWALLOWED: Rinse mouth. Do NOT induce vomiting P363 : Wash contaminated clothing before reuse P304 + P340 + P310 : IF INHALED: Remove person to fresh air and keep comfortable for breathing. Immediately call a POISON CENTER/doctor P305 + P351 + P338 + P310 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. Immediately call a POISON CENTER/doctor P405 : Store locked up. |
| Signal word | Danger |
Transport information
| Category | 8 |
|---|---|
| Packaging category | III |
| UN number | UN1759 |
Product Category
Chemical structure
Chemicals by Class Compounds by Element Classes Metal Compounds [Chemical Structural Class] Hf (Hafnium) Compounds [Chemical Structural Class] Transition Metal Compounds [Chemical Structural Class] Sulfur Compounds [Chemical Structural Class] Trifluoromethanesulfonates [Chemical Structural Class] Trifluoromethanesulfonic Acid Salts [Chemical Structural Class]
Chemistry Catalysis and Inorganic Chemistry Transition Elements [Catalysis and Inorganic Chemistry] Hafnium [Catalysis and Inorganic Chemistry] Synthetic Reagents Acids, Bases [Synthetic Reagents] Acids [Synthetic Reagents] Stable Lewis Acids in Aqueous Media [Synthetic Reagents] Lewis Acids [Synthetic Reagents]